Highest Quality Standards
Genomics is committed to the highest quality standards for our products and services so our customers always receive the best. Our commitment to quality is demonstrated by our stringent internal processes, using the most advanced technology, and our accreditation in ISO 9001, ISO13485, CAP, CLIA, and cGMP FDA 3009882691 for the manufacture of ASRs (Analyte Specific Reagents) for use in IVD products.
Click the icons below to view our accreditation documentation or it will redirect to further information on the accreditation.
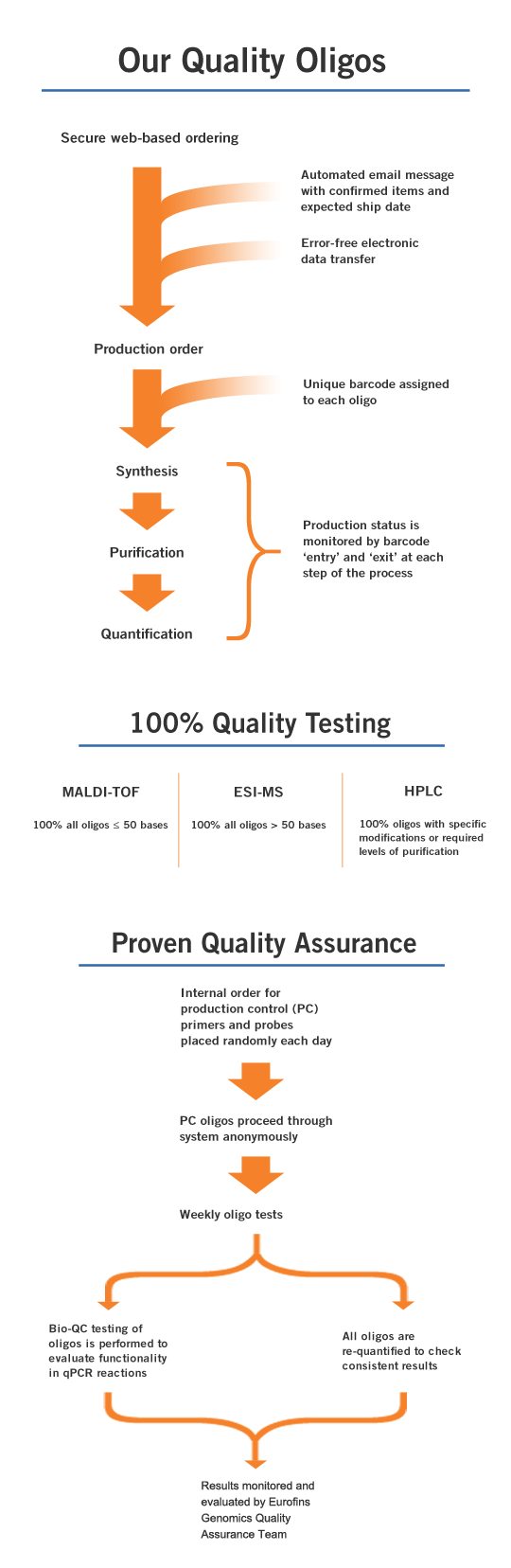
100% QC on Every Oligo
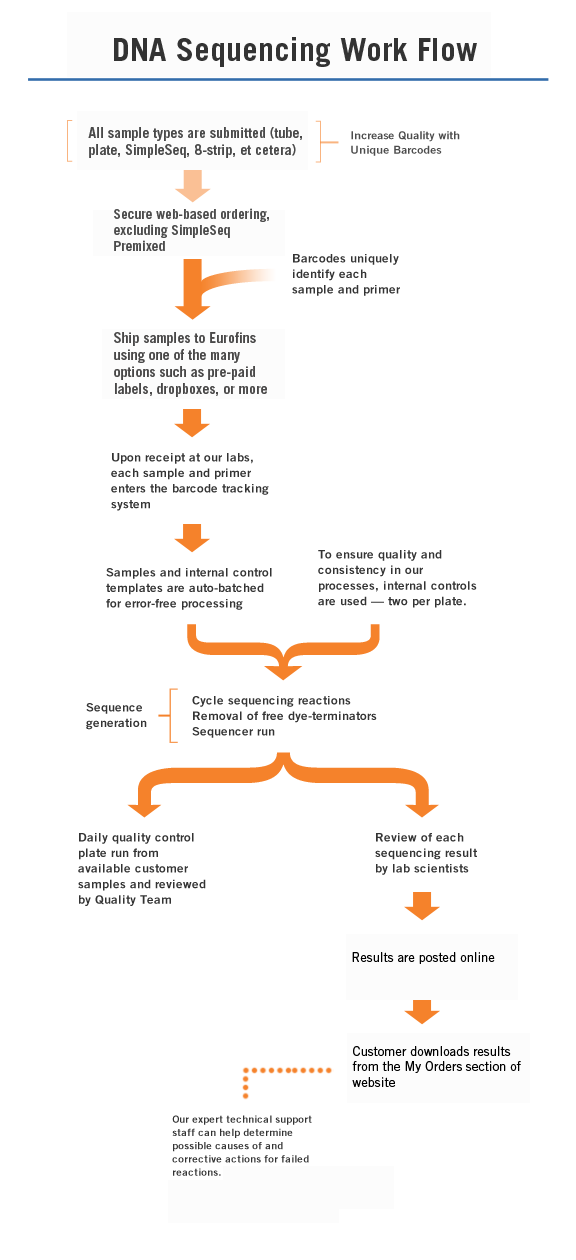
Eurofins Genomics performs quality control on every oligo that flows through our state-of-the-art facility, using electrospray ionization (ESI) Mass Spectrometry. In fact, we were the first oligonucleotide company to pioneer 100% MS testing back in 1998. It is provided as a free service to all customers. MS verifies the sequence by molecular weight and provides a visual peak trace for a qualitative measure of quality.
For information on custom quality control for commercial or special projects, visit the custom QC section.