Special Protocols (SP)
Formulations, Commercialization, Packaging, and Kitting
Companies operating in the commercial sector, such as molecular diagnostics, synthetic biology, NGS applications, CRISPR, and therapeutics, typically have additional needs that go beyond our standard workflow. Eurofins Genomics is committed to delivering the highest degree of quality, service, and satisfaction to industrial customers pursuing commercialization. These projects typically involve a high order volume and originate from a commercial product launch.
We have developed a wide array of special protocols to ensure optimal results and ultimately fuel your growth trajectory. Some of these special protocols require discussion and planning before starting production, while others can be ordered online through our DNA plate order page. New customers
Select from the following options to proceed
- Special Project request (email link) to discuss the scope, requirements, goals, and production workflow required for success on the project. (High level of complexity or new projects.)
- Online Ordering for Special Protocols Select special protocols from the DNA plate order page. (Existing customers or low complexity.)
- Additional Information
Current Online Options for Special Protocols
The following list is subject to change. Furthermore, it does not account for all special protocols offered by our lab, but is intended to capture common requests typically placed on a reorder for bulk material.
- GMP: Commercialization of a product requires companies to adhere to high regulatory standards, all the way through to their suppliers. Eurofins Genomics has been approved by the FDA for cGMP manufacture of ASRs (Analyte Specific Reagents) for use in IVD products. New customers should contact us to discuss their GMP project before selecting this option on the website. GMP projects typically require several meetings between the supplier and client before production can begin, so that both parties can assess the requirements of the project and prepare. For existing customers seeking to reorder bulk material for a pre-defined project, the online option is very helpful to streamline the ordering process. Learn more about GMP.
- Pooling and Multiplexing: Mixing multiple sequences together is a novel requirement within the industrial sector, especially when targeting many different regions within one assay. These pools can range from a single plate up to hundreds of thousands of oligos. Learn more on pooling (tab 4 of new page)
- Custom Yield: This protocols ensures a guaranteed yield is met on the order.
- Additional Concentration Check: Additional concentration checks help to ensure the exact yield, volume, and concentration is present in the final deliverable. Commercial assays, to give one example, require highly specific concentrations and may need a second concentration check. The normal acceptable variance may not be sufficient.
- Stamping: Stamping involves pipetting out small amounts of a source material into multiple, secondary plates at a specific concentration.
- Special Normalization Requirements: this protocol is used to assign customized normalization requirements to an order.
Additional protocols are available by request, suc has custom HPLC columns, custom plate types, annealing, expedited processing, and more.
In the past, all special projects were managed outside of the eCommerce platform due to the level of complexity, customization, and hands on attention required. However, Eurofins Genomics recently added an option on the DNA oligo plate order page that allows customers to select special protocols when ordering. This option is mainly geared for companies who have already discussed the specifications of their project with us and need to order additional material on a regular basis. In this regard, the online options offer a convenient solution to capture additional services throughout the entire process.
Restrictions
- Not allowed for small volume orders. Due to the cost and labor involved, these services are designed for commercial companies and custom projects, not small orders.
- Complex projects may require a meeting with the customer to further understand their needs and the project scope.
- If you currently enter an ISOP number in the order notes, please continue to do so. These options do not replace the ISOP.
Pricing
Every project is different. This means the scope, effort, and complexity can vary dramatically between different projects. For this reason, we designed the online protocol pricing around two principles. 1.) For simpler protocols, pricing will scale with the size of the order. 2.) For more complex protocols, the price will appear as “n/a” and the customer needs to submit the order as a quote for review.
Click the price details link in the cart to view the price of those special protocols.
How to Order Online
- Go to the DNA plate order page.
- On step 2, select “yes’ beside special protocols and select which protocols you wish to add to the order.

- Complete the remaining steps on the order page.
- Go to the cart. The price will appear as “n/a” due to the inherent complexity involved in special projects. The order must be submitted as a quote for manual pricing.
- Click the quote button instead of checkout.

- Submit the quote. These steps mirror the normal checkout process You will not be charged until you decide to convert the quote into an order.
- The technical support team will call you to gather information on the project.
- Once the project scope is clear, our technical support team will complete the quote and published it back to the website.
- Go to the My Quotes page and click the accept button.
- Checkout the order.
You are searching for a partner, not just a supplier. It is key to understand that principle. You are about to embark on an exciting journey to develop an assay, kit, or commercial product which could save lives, improve healthcare, or even reshape the world. It will be exhilarating but also stressful. Each milestone will be defined by challenges. Fortunately, Eurofins Genomics is built to alleviate that stress and help you overcome challenges. We are the partner that will go the extra mile to make your assay perform to its highest level.
The commercialization process is shown below. It begins with research and ends with the commercial launch of a product. Each phase has different needs, which Eurofins Genomics is prepared to deliver on. For instance, the research phase typically involves smaller orders with experimental sequence designs. The sequences can sometimes be challenging and exciting to work on. Meanwhile, the commercial phase involves ramping up to larger amounts of material while ensuring quality never diminishes.
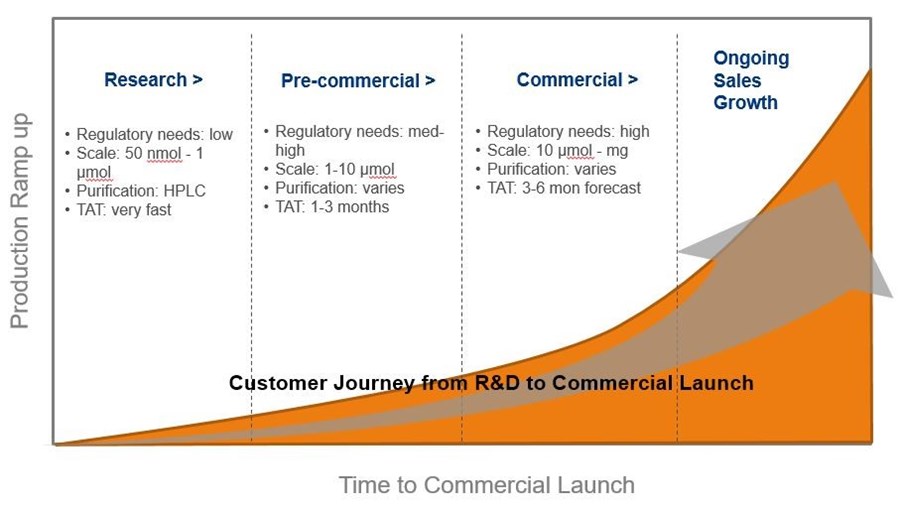
Science evolves. Companies must evolve with it. As our customers evolve to meet the demands of novel research fields such as diagnostics, NGS, CRISPR, and therapeutics, Eurofins Genomics is right beside them—innovating, expanding, and preparing new services to accelerate your product’s commercialization.
Industries
A wide variety of industries utilized these special protocols, but the key sectors include diagnostics (Dx), NGS, CRISPR, synthetic biology, biotech, and therapeutics.
These sectors require a higher degree of customization from suppliers due to the sensitivity of equipment, regulatory requirements, and supply chain needs. No matter the industry, Eurofins Genomics is commited to providing the highest quality and service for your products.
Types of Products
- Research and Pre-commercial--includes RUO (research use only) and IUO (investigational use only).
- Lab Developed Tests (LDT)--a complete In Vitro test produced in a single laboratory.
- Companion Diagnostics (CDx)—designed to be used in conjunction with treatment by a physician.
- Commercial Product—FDA approved product to be used with patient samples.
Categories
The range of services can vary dramatically but a majority fall into one or more of the following categories. Further detail can be found in the previous tab on ordering online.
- Customization to the overall production workflow, including oligo synthesis, liquid handling, quality control, and purification.
- Scale—commercialization involves synthesizing at higher scales, which is typically delivered in bulk lots.
- Purity—due to the sensitivity of NGS equipment, many commercial assays require high purification.
- Regulatory requirements and GMP.
- Pooling, annealing, and multiplexing after synthesis is completed.
- Integration with LIMS and/or IT systems.
- Formulation to reach specific concentration and volume levels.
- Quality control including both additional QC methods and additional QC checks.
- Packing and kitting
Industry Needs
NGS technology is the catalyst behind much of the explosive growth within the life science industry over the past decade. The decrease in cost and rise in the portability of DNA sequencers, combined with the development of NGS techniques, has provided high throughput analysis and sequencing of genomic data. NGS oligonucleotide components include NGS adaptors/indexes, UDIs, barcoded adaptors, targeted enrichment panels, and hybridization capture probes for targeted sequencing. These demanding NGS applications require rigorous manufacturing processes due to the ability to detect single molecules. Purity, as it pertains to oligos, is especially important to avoid adapter cross-contamination that will lead to misalignment in sequencing results
Furthermore, custom oligonucleotides are a critical component of molecular diagnostic assays. Almost any assay developed today requires oligos, specifically probes, and primers. Furthermore, companies require consistent quality between production batches since the oligos determine the performance parameters of their experiment, such as the detection limit and specificity of their assay. In summary, MDx customers need an oligonucleotide manufacturing process that is GMP compliant for their commercial products.
Scaling up
Scale up, sometimes referred to as the “ramp up” phase, refers to the supplier taking an MDx assay from small-scale RUO to large-scale manufacturing. Preserving product quality and functional performance through this period is critical. The end goal is to produce single batch sizes to the customer’s yield specifications.
The typical requirement is to have a single production batch that produces the yield requirement that typically ranges from 1.0 µmole to multiple grams.
Regulatory / QC / QA /Traceability
One of the most important criteria for a large commercial company is batch-to-batch reproducibility so their assay performs exactly the same with each lot. All aspects of our manufacturing process are monitored and documented resulting in a formal batch record. Our QA teams will support your vendor qualifications, paper audits, on-site audits, and the overall product release of GMP orders. In some cases, a certificate of analysis must be signed before the product can be released by the QA team.
Packaging and Kitting
Depending on customer’s market strategy, they may need Eurofins Genomics, the oligo supplier, to ship the material in customized packages. This process can include additional services such as kitting and distributing to the end customer as well.