Science evolves. Companies must evolve with it. As our customers evolve to meet the demands of novel research fields such as diagnostics, NGS, CRISPR, and therapeutics, Eurofins Genomics is right beside them—innovating, expanding, and preparing new services to accelerate your product’s commercialization.
A Partner for Commercial Companies
You are searching for a partner, not just a supplier. It is key to understand that principle. You are about to embark on an exciting journey to develop an assay, kit, or commercial product which could save lives, improve healthcare, or even reshape the world. It will be exhilarating but also stressful. Each milestone will be defined by challenges. Fortunately, Eurofins Genomics is built to alleviate that stress and help you overcome challenges. We are the partner that will go the extra mile to make your assay perform to its highest level.
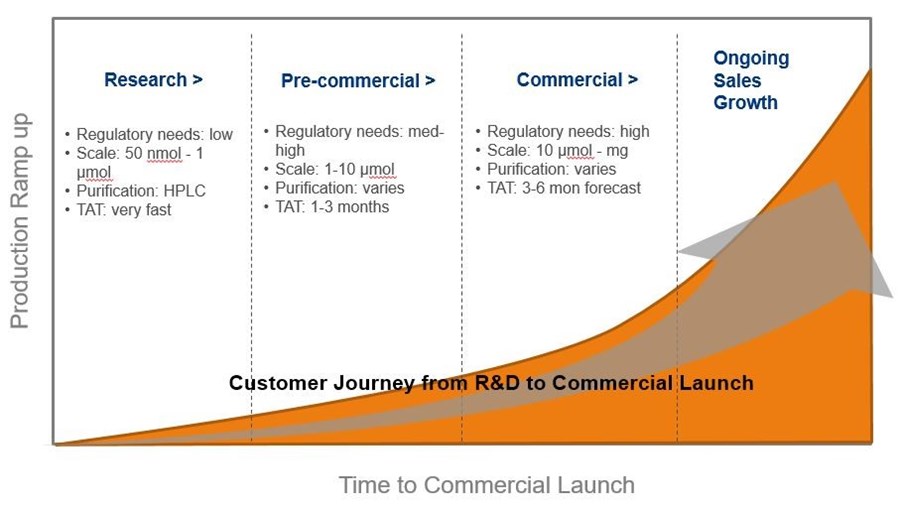
The commercialization process is shown below. It begins with research and ends with the commercial launch of a product. Each phase has different needs, which Eurofins Genomics is prepared to deliver on. For instance, the research phase typically involves smaller orders with experimental sequence designs. The sequences can sometimes be challenging and exciting to work on. Meanwhile, the commercial phase involves ramping up to larger amounts of material while ensuring quality never diminishes.
Industries
A wide variety of industries utilized these special protocols, but the key sectors include diagnostics (Dx), NGS, CRISPR, synthetic biology, biotech, and therapeutics.
These sectors require a higher degree of customization from suppliers due to the sensitivity of equipment, regulatory requirements, and supply chain needs. No matter the industry, Eurofins Genomics is committed to providing the highest quality and service for your products.
Types of Products
- Research and Pre-commercial--includes RUO (research use only) and IUO (investigational use only).
- Lab Developed Tests (LDT)--a complete In Vitro test produced in a single laboratory.
- Companion Diagnostics (CDx)—designed to be used in conjunction with treatment by a physician.
- Commercial Product—FDA approved product to be used with patient samples.
Categories
The range of services can vary dramatically but a majority fall into one or more of the following categories. Further detail can be found in the previous tab on ordering online.
- Customization to the overall production workflow, including oligo synthesis, liquid handling, quality control, and purification.
- Scale—commercialization involves synthesizing at higher scales, which is typically delivered in bulk lots.
- Purity—due to the sensitivity of NGS equipment, many commercial assays require high purification.
- Regulatory requirements and GMP.
- Pooling, annealing, and multiplexing after synthesis is completed.
- Integration with LIMS and/or IT systems.
- Formulation to reach specific concentration and volume levels.
- Quality control including both additional QC methods and additional QC checks.
- Packing and kitting
Industry Needs
NGS technology is the catalyst behind much of the explosive growth within the life science industry over the past decade. The decrease in cost and rise in the portability of DNA sequencers, combined with the development of NGS techniques, has provided high throughput analysis and sequencing of genomic data. NGS oligonucleotide components include NGS adaptors/indexes, UDIs, barcoded adaptors, targeted enrichment panels, and hybridization capture probes for targeted sequencing. These demanding NGS applications require rigorous manufacturing processes due to the ability to detect single molecules. Purity, as it pertains to oligos, is especially important to avoid adapter cross-contamination that will lead to misalignment in sequencing results
Furthermore, custom oligonucleotides are a critical component of molecular diagnostic assays. Almost any assay developed today requires oligos, specifically probes, and primers. Furthermore, companies require consistent quality between production batches since the oligos determine the performance parameters of their experiment, such as the detection limit and specificity of their assay. In summary, MDx customers need an oligonucleotide manufacturing process that is GMP compliant for their commercial products.
Scaling up
Scale up, sometimes referred to as the “ramp up” phase, refers to the supplier taking an MDx assay from small-scale RUO to large-scale manufacturing. Preserving product quality and functional performance through this period is critical. The end goal is to produce single batch sizes to the customer’s yield specifications.
The typical requirement is to have a single production batch that produces the yield requirement that typically ranges from 1.0 µmole to multiple grams.
Regulatory / QC / QA /Traceability
One of the most important criteria for a large commercial company is batch-to-batch reproducibility so their assay performs exactly the same with each lot. All aspects of our manufacturing process are monitored and documented resulting in a formal batch record. Our QA teams will support your vendor qualifications, paper audits, on-site audits, and the overall product release of GMP orders. In some cases, a certificate of analysis must be signed before the product can be released by the QA team.
Packaging and Kitting
Depending on customer’s market strategy, they may need Eurofins Genomics, the oligo supplier, to ship the material in customized packages. This process can include additional services such as kitting and distributing to the end customer as well.