Custom Oligo Technology
Eurofins Genomics uses optimized phosphoramidite chemistry and reagents of the highest quality. Proprietary synthesis platforms provide computer-controlled oligo synthesis, cleavage, deprotection, and desalting in a parallel processing format.
Eurofins Genomics oligonucleotides are synthesized with a high coupling efficiency (typically >99%) under salt-free conditions, avoiding the need for additional purification for most molecular biology applications, such as PCR, RT-PCR, sequencing, and hybridization studies.
The high coupling efficiency of Eurofins Genomics proprietary synthesis technologies allows efficient synthesis of longmers up to 100 bases at high yields. In addition, a large number of modifications are available. All oligos are subject to stringent quality control (QC).
Synthesis Cycle
The synthesis takes place on a solid support -- controlled pore glass (CPG) -- where the 3'-nucleoside of the oligo being synthesized is attached. The oligonucleotide synthesis starts with the 3’ base. During the synthesis cycle the oligonucleotide is elongated toward the 5’ end.
For each coupling step, the following nucleotide is delivered as a nucleoside phosphoramidite where a reactive phosphoramidite group is located at the 3’-OH and the 5’-OH is modified with a dimethoxytrityl protection group (DMT).
The reactive phosphoramidite group reacts with the 5’-OH of the attached oligonucleotide.
The oligonucleotide synthesis cycle comprises the following steps:
- Detritylation:
The cleavage of the DMT protecting group from the previous base to form a 5’ reactive hydroxyl function.
- Coupling:
The 5’-OH group reacts with added activated phosphoramidite bearing the next base. As a result, both nucleosides are linked together.
- Capping:
The yield of the coupling step is - as every chemical reaction - less than 100%. Therefore any free 5’-OH groups which did not couple to the next nucleotide have to be excluded from the next coupling steps. Acetylation is used in the capping step to block all reactive 5’-OH groups.
- Oxidation:
The nucleotides are linked via phosphorous containing bonds that have been created in the coupling step. The phosphorus group is oxidized using iodine solution.
After the oxidation step a new synthesis cycle starts to add the next nucleotide (see step 1). The cycle is repeated until the desired sequence is synthesized.
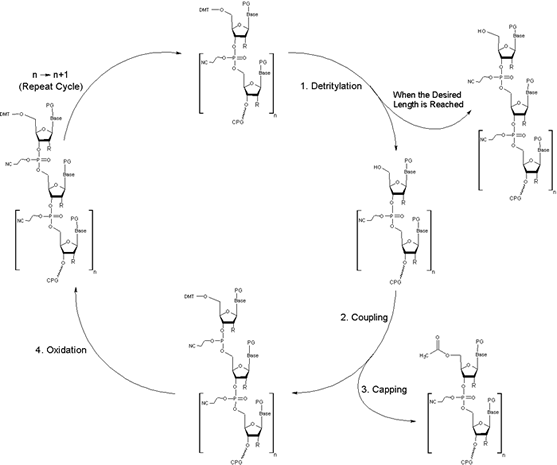
PG=Protecting Group; R depends on the type of nucleic acid being synthesized.
Click on image for larger view.
Deprotection:
After the synthesis has been performed and the desired length has been reached, the oligo undergoes one last detritylation reaction. The oligo is then cleaved from the solid support and the remaining protecting groups are cleaved to yield a biologically functional oligonucleotide.
How Modifications are Attached to an Oligo
Oligos can be modified in several different ways by utilizing the active groups of the nucleotide or creating nucleotide analogues. The most common oligo modifications are listed next:
- Terminal modifications utilizing the 3' and 5' OH groups
(e.g., C6 and C7 amino modifiers, biotin-ON, biotin-TEG, cholesterol-TEG, fluorescein, thiol modifications, phosphate)
- Base modifications
(e.g., 5-bromo-dU, 5-bromo-dC, 5-fluoro-dU, deoxyinosine, 5-iodo-dC, 5-iododU, 5-methyl-dC, 5-nitroindole, deoxyuridine)
- Thymidine analogues, replacing a T residue in the sequence
(e.g., C2 dT and C6 dT amino modifiers, biotin-dT, dabcyl-dT, fluorescein-dT, TAMRA-dT)
All the previously listed modifications are available internally and on the 5' end. Some are also available on the 3' end. Most 3' modifications require special controlled pore glass (CPG) columns, which are not available for all modifications.
- Post-synthesis modifications
Modifications are attached post-synthetically, via an amino modifier (e.g., coumarin, digoxygenin, Oregon Green®, ROX, Texas Red®-X, BODIPYdyes). By choosing the appropriate amino modifier, these modifications can be attached in different positions in the oligo.
- Modifications of the phosphate group
(e.g., phosphorothioation)
- 2' Modifications
(2'-O-methyl A/C/G/U, ribo A/C/G/U)
NOTE: Unmodified oligos are very stable molecules; however, with the addition of modifications, their properties can change significantly. Modified oligos are more sensitive to light, pH, and frequent freezing and thawing. Proper storage and handling of modified oligos is imperative.